Gene signatures in patients with early breast cancer and relapse despite pathologic complete response | npj Breast Cancer

Neoadjuvant and adjuvant treatment of patients with HER2-positive early breast cancer - ScienceDirect
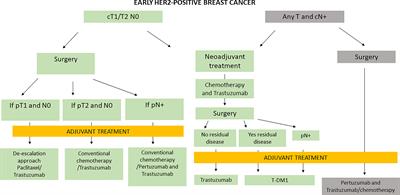
Frontiers | Management of HER2-Positive Early Breast Cancer in Italy: A Maze Presenting Opportunities and Challenges

Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer - ScienceDirect

OncoAlert on X: "The #OncoAlertTopTweet 🚨Day5⃣of #ESMO21 Tweet @Dr_Ivanoncologo Dr. Harbeck: Predictive impact of biomarkers🎯on pCR and survival after de-escalated neoadjuvant T-DM1 w/ or w/o endocrine therapy (ET) vs Trastuzumab🧪+ ET in
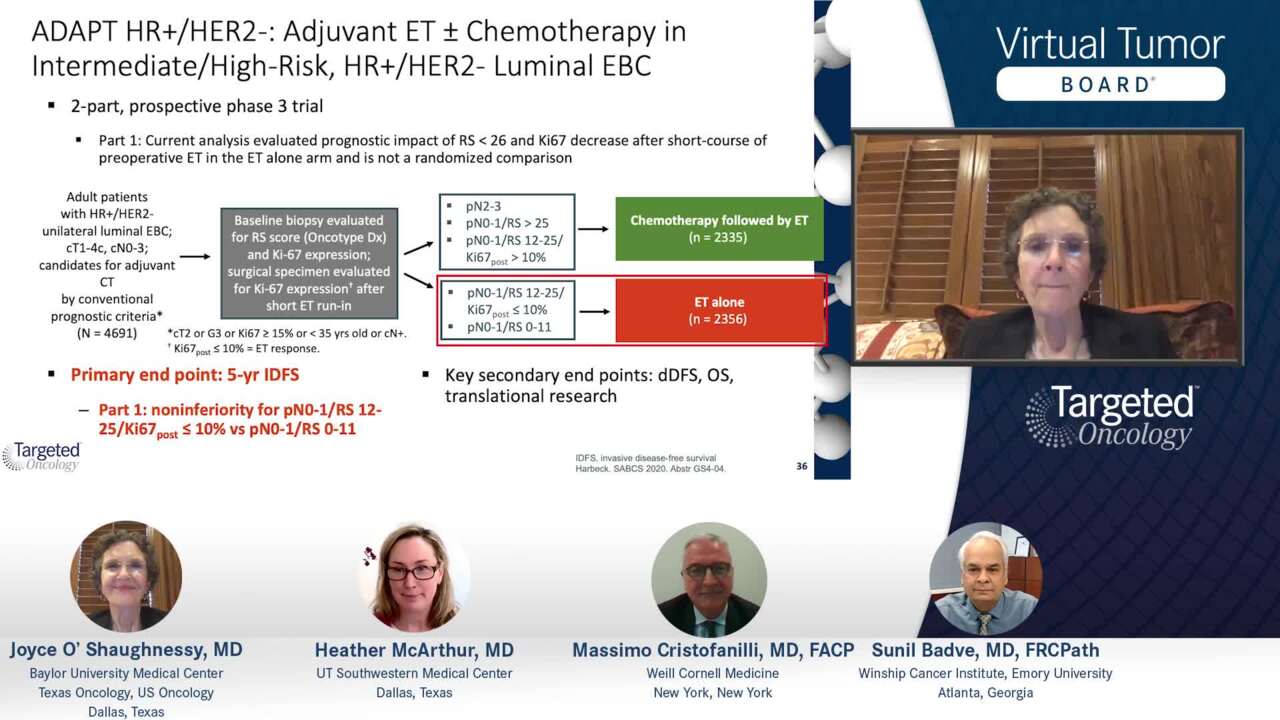
WSG ADAPT – adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator ...

Molecular and protein markers for clinical decision making in breast cancer: Today and tomorrow - ScienceDirect

Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial - The Lancet Oncology

PDF) WSG ADAPT - adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: Study protocol for a prospective, multi-center, controlled, non-blinded, randomized ...

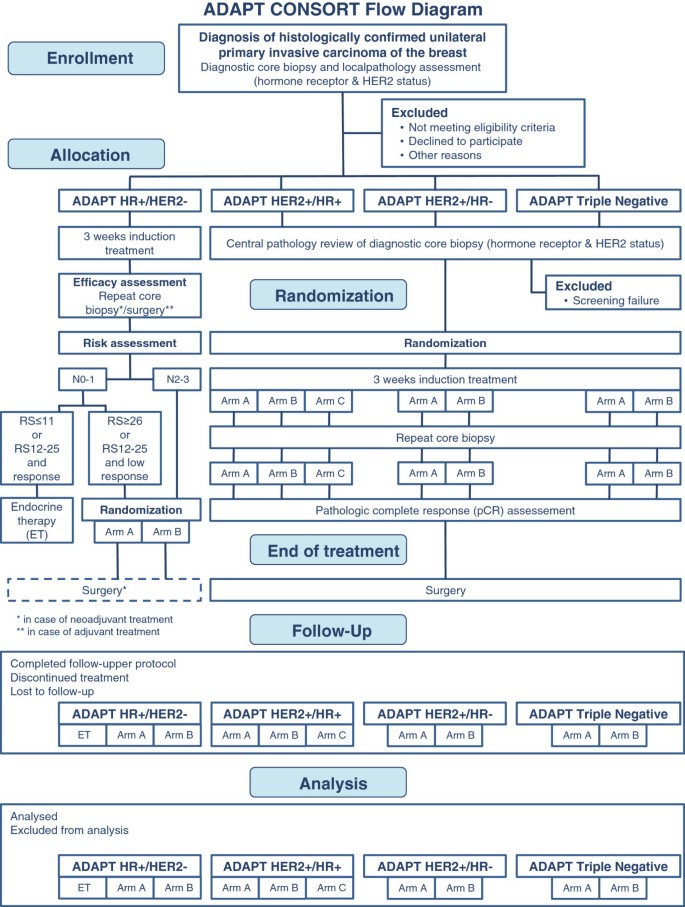
CONSORT diagram. Disposition of patients in the ADAPT (Adjuvant Dynamic... | Download Scientific Diagram

ADAPT HER2+/HR+ trial design. HER2+/HR+ patients receive either T-DM1... | Download Scientific Diagram

ADAPT HER2+/HR- trial design. HER2+/HR- patients receive either dual... | Download Scientific Diagram

Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures | PNAS

De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR–): survival outcomes from a multicentre, open-label, randomised, phase 2 ...

De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR–): survival outcomes from a multicentre, open-label, randomised, phase 2 ...

Susan G. Komen on X: "WSG ADAPT HR-/HER2+ phase II trial showed improved pathological complete response (pCR) and survival in patients treated by #de-escalated chemotherapy, pertuzumab, and trastuzumab. Early pCR strongly associated
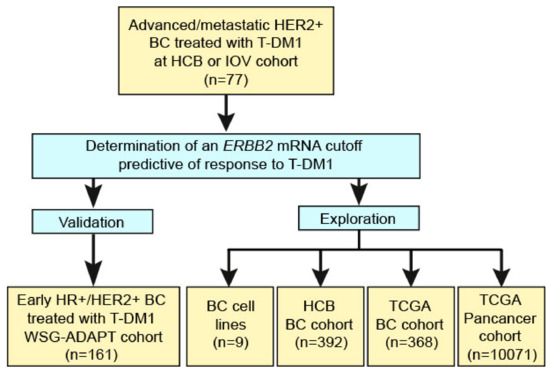
Cancers | Free Full-Text | ERBB2 mRNA Expression and Response to Ado-Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer
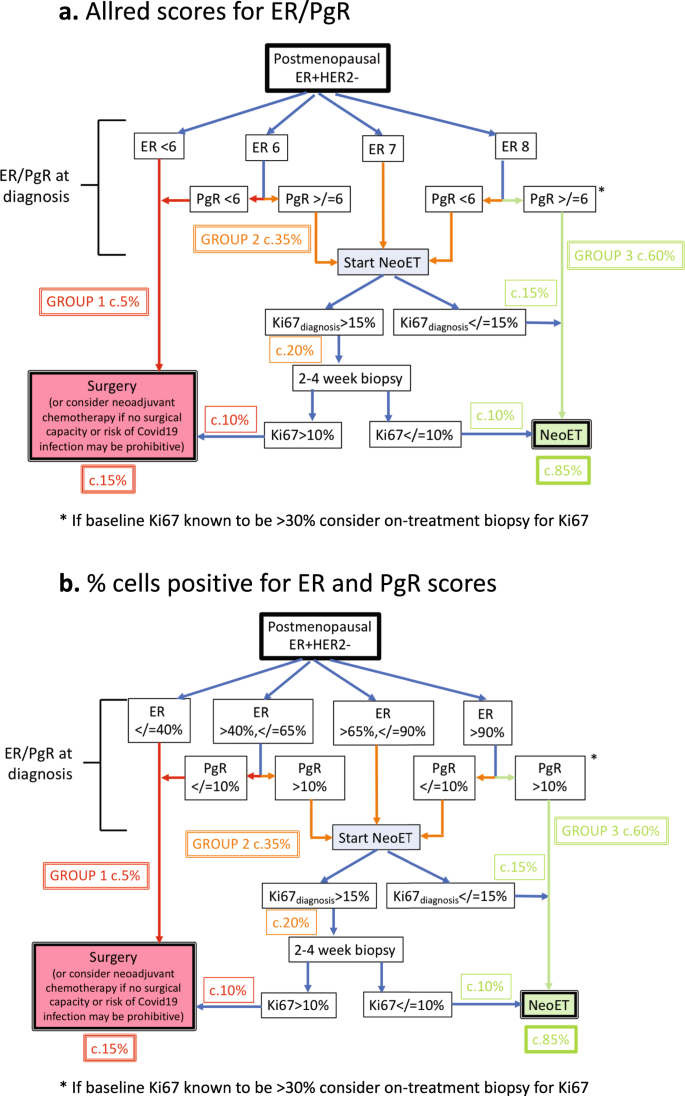
Evidence-based guidelines for managing patients with primary ER+ HER2− breast cancer deferred from surgery due to the COVID-19 pandemic | npj Breast Cancer
S5-03 Final analysis of WSG-ADAPT HER2+/HR+ phase II trial: Efficacy, safety, and predictive markers for 12-weeks of neoadjuvant

De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR–): survival outcomes from a multicentre, open-label, randomised, phase 2 ...