Adaptive Biotech on X: "PRESS RELEASE: Adaptive announces translational collaboration with @TakedaPharma to measure minimal residual disease (MRD) with its clonoSEQ® Assay across Takeda's hematologic malignancy pipeline. https://t.co/UKraSc7uIX https ...
Integrated analysis of next generation sequencing minimal residual disease ( MRD) and PET scan in transplant eligible myeloma patients | Blood Cancer Journal

Adaptive and Genentech Partner to Use clonoSEQ® Assay to MRD in Study of Chronic Lymphocytic Leukemia Patients

SparkCures - Adaptive recently announced an exciting new update to their clonoSEQ MRD (Measurable Residual Disease) testing service. Blood collections are now available to clonoSEQ patients at home or through LabCorp® Patient

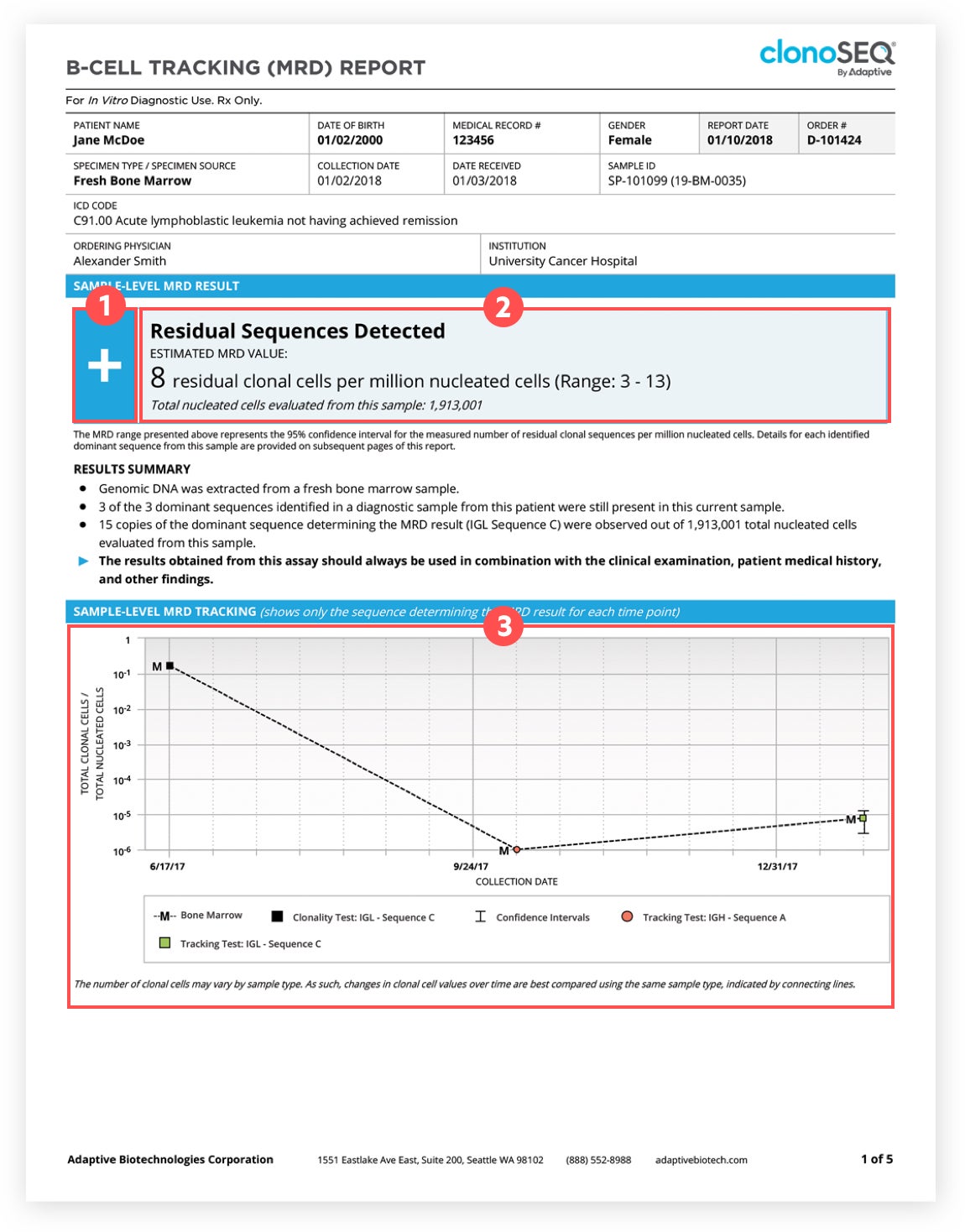
FLASCO / The clonoSEQ® Assay is now FDA-cleared and covered by Medicare for detecting and monitoring MRD in bone marrow samples from patients with multiple myeloma or B-cell ALL.
Analytical evaluation of the clonoSEQ Assay for establishing measurable (minimal) residual disease in acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma | BMC Cancer | Full Text

Adaptive Biotechnologies Corp. on LinkedIn: Sr. Medical Director Allison Jacob discusses the recent Blood Cancer…
La FDA accorde la désignation De Novo pour le test clonoSEQ d'Adaptive Biotechnologies servant à détecter et à surveiller la maladie résiduelle minimale (MRM) chez les patients atteints de myélome multiple et

clonoSEQ is now FDA-cleared to assess MRD in patients with CLL | We're excited to announce expanded FDA clearance for the clonoSEQ Assay to assess Minimal Residual Disease (MRD) in patients with

The relevance of MRD assessment in the clinical management of Multiple Myeloma (MM) patients - YouTube

Dara-KRd, Autologous Transplantation and MRD Response-Adapted Consolidation and Treatment Cessation. Final Primary Endpoint Analysis of the MASTER Trial




%20Testing%20Market.png)